Institutional dashboards on clinical trial transparency for University Medical Centers: A case study | PLOS Medicine
![PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/62bcc7885dac29517026f3ba577045d3b93dbb7b/2-Table2-1.png)
PDF] Comparison of Eligibility Criteria Between Protocols, Registries, and Publications of Cancer Clinical Trials. | Semantic Scholar

Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers | The BMJ

Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles – The Publication Plan for everyone interested in medical writing, the development of medical publications, and publication planning

Publications | Free Full-Text | Leveraging Open Tools to Realize the Potential of Self-Archiving: A Cohort Study in Clinical Trials

Race and ethnicity representation in clinical trials: findings from a literature review of Phase I oncology trials | Future Oncology
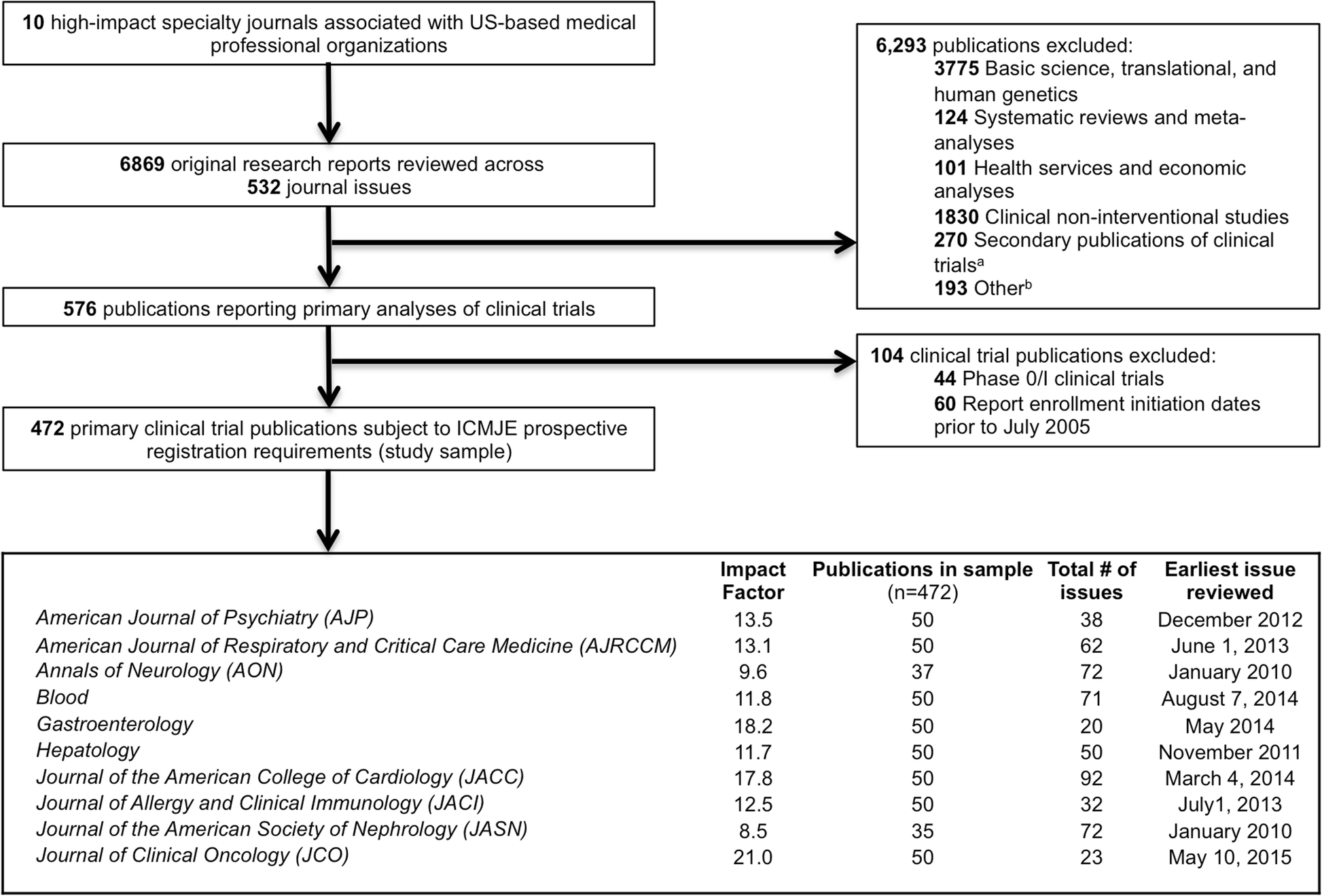
Adherence to the International Committee of Medical Journal Editors' (ICMJE) prospective registration policy and implications for outcome integrity: a cross-sectional analysis of trials published in high-impact specialty society journals | Trials
Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups | The National Academies Press
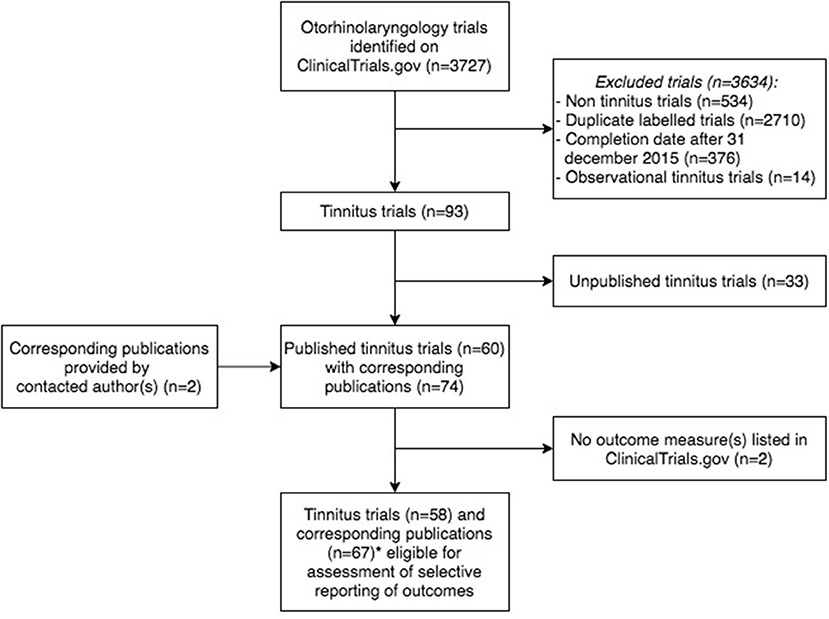
Frontiers | Selective Reporting of Outcomes in Tinnitus Trials: Comparison of Trial Registries With Corresponding Publications

Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers | The BMJ

TranspariMED on Twitter: "THREAD: Today we publish a report comparing: (a) what WHO says funders should do (b) what clinical trials funders actually do Let's start with the 11 safeguards against research