
REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

Good Clinical Practice: A Question & Answer Reference Guide, May 2018: Earl W. Hulihan, Earl W. Hulihan: 9780996346252: Amazon.com: Books

Final ICH GCP E6 R2 Addendum: Overview of Changes Impacting Sponsors/CROs/ Clinical Investigator/Site - YouTube
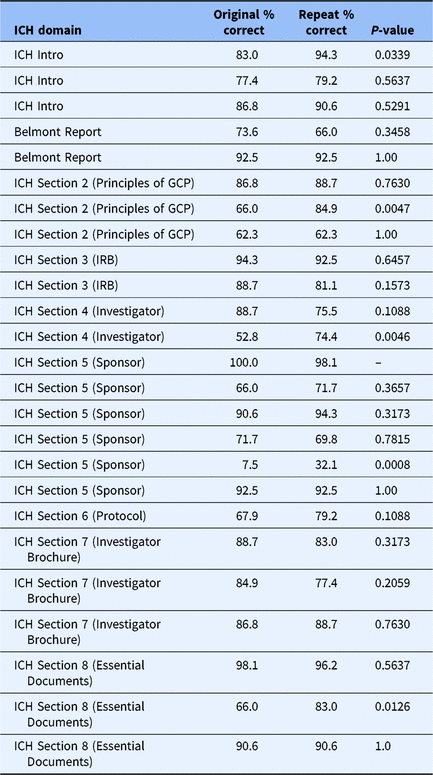
Creating and testing a GCP game in an asynchronous course environment: The game and future plans | Journal of Clinical and Translational Science | Cambridge Core









.jpg)






