Addendum to the Guidance on the Management of Clinical Trials during the COVID-19 (Coronavirus) pandemic Date: 25.3.2020 Versio
NOTIFICATION OF A SUBSTANTIAL AMENDMENT TO A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR HUMAN USE TO THE COMPETENT AUTHORITIES AN
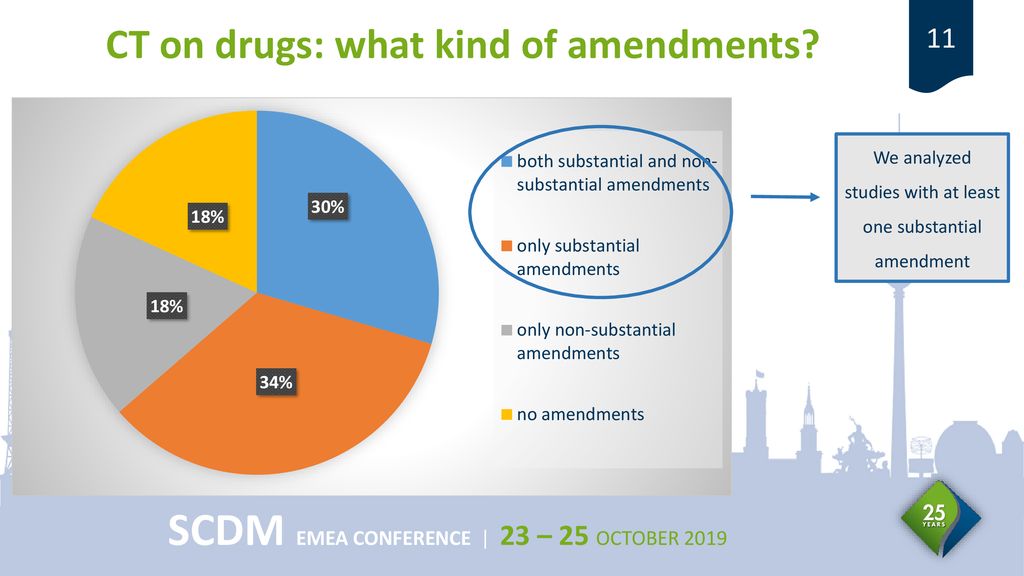
49 Annex 2: Substantial Amendment Form NOTIFICATION OF A SUBSTANTIAL AMENDMENT TO A CLINICAL TRIAL ON A MEDICINAL PRODUCT FOR

EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL. Brussels, ENTR/F2/BL D(2003) CT 1 Revision 2 - PDF Free Download
1. Name of service Approval for the substantial amendment (to a clinical trial on a medicinal product for human use) 2. Recipien
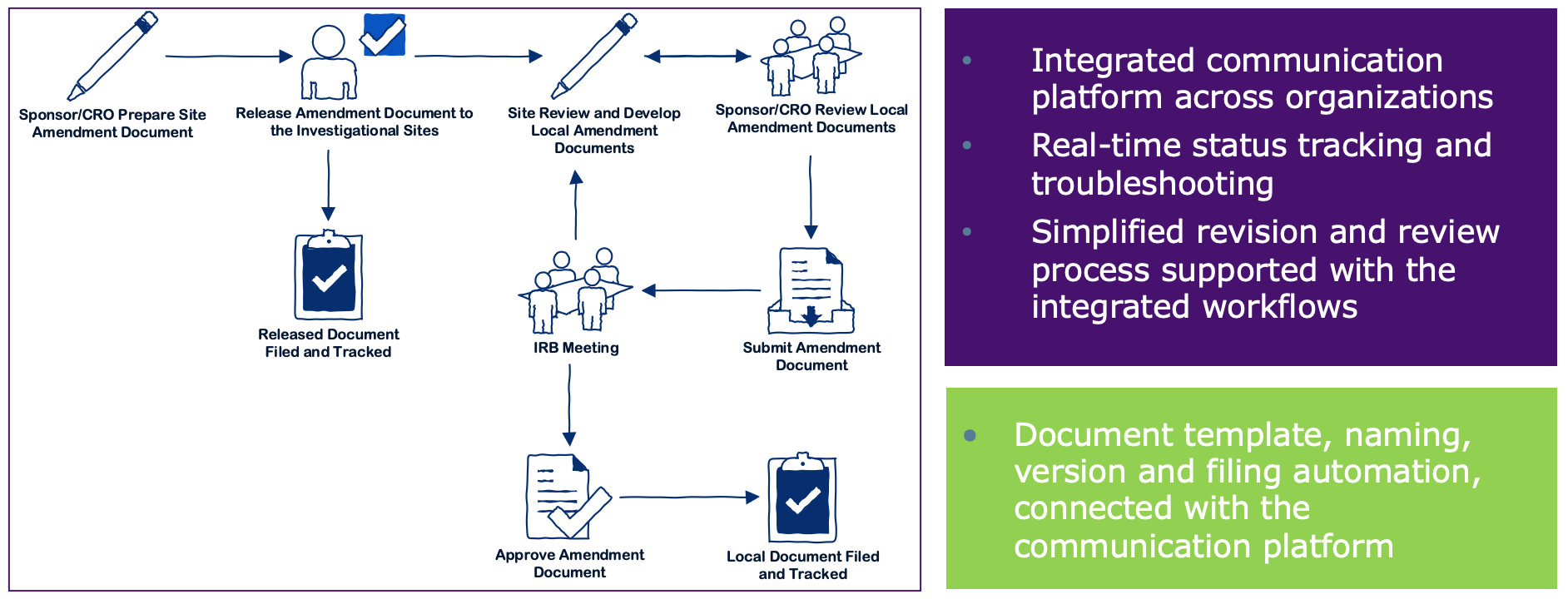
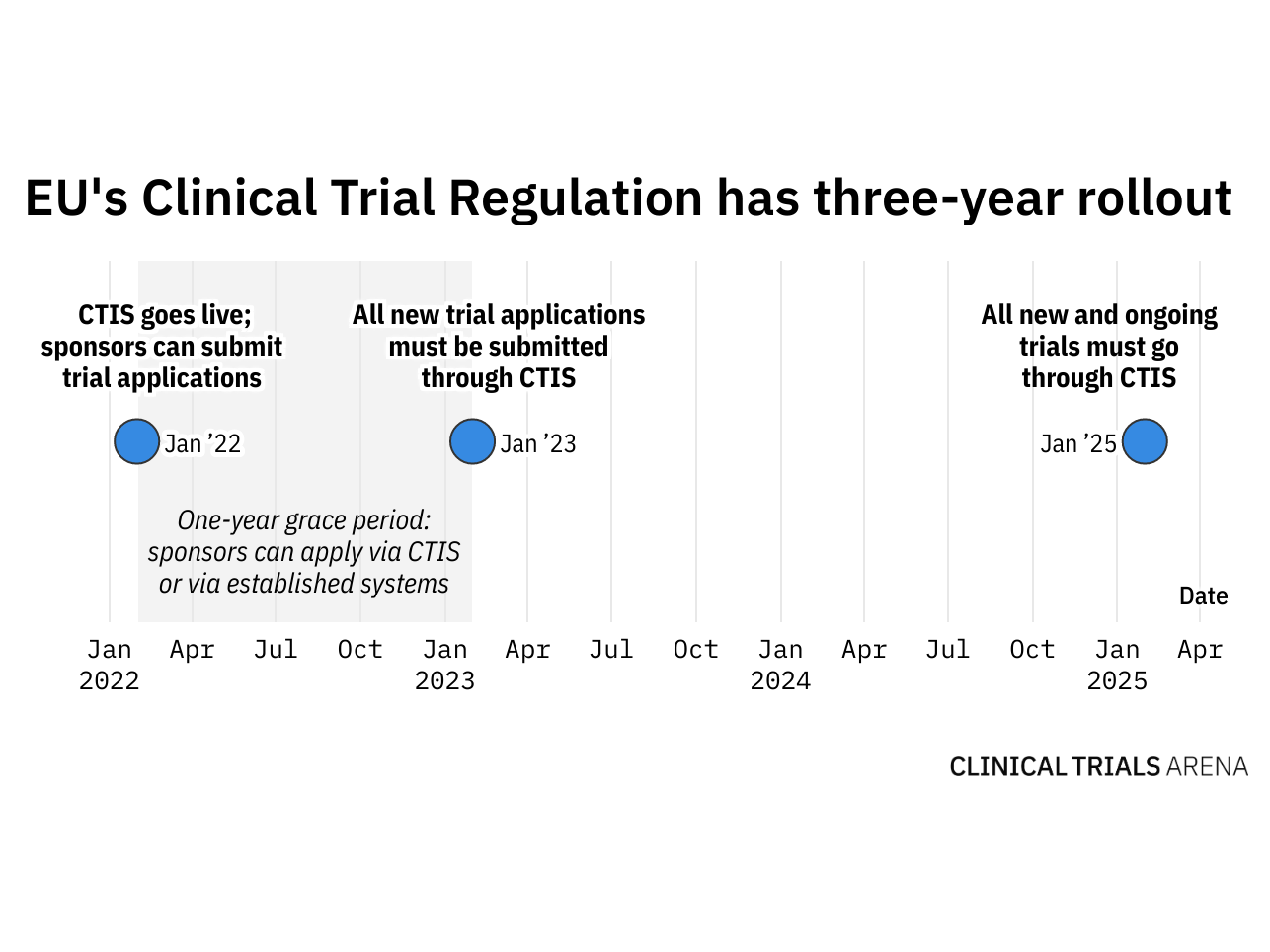
Guide to Clinical Trials Regulation-National Collaboration Project (CTR-NCP) Health Products Regulatory Authority and National